
| Home | Syllabus | Schedule | Lecture Notes | Extras | Glossary |

| Home | Syllabus | Schedule | Lecture Notes | Extras | Glossary |
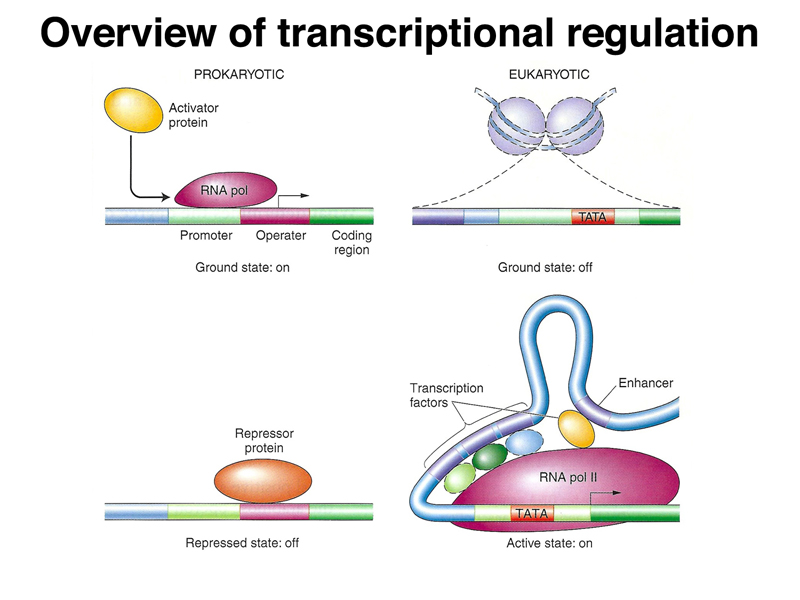
We began by once again comparing prokaryotic and eukaryotic transcriptional regulation. As shown below, in prokaryotes, the genome is essentially naked DNA with no barrier to transcription. While there are some activator proteins, like the CAP protein, that can increase the basal level of transcription under some circumstances, the default state of the prokaryotic genome is on. Repressor proteins, like the lac and trp repressor, are used to prevent transcription except under the right metabolic conditions (lactose but no glucose in the case of the lac operon, and low tryptophan levels in the case of the trp operon). In eukaryotes, the genome is packed into nucleosomes, so the ground state of transcription is off. In order for genes to be expressed, DNA must be partially unpacked from nucleosomes. Most transcriptional regulation in eukaryotes is positive: transcriptional activators that are part of a very large and complicated transcription initiation complex are the key elements of eukaryotic transcriptional regulation.
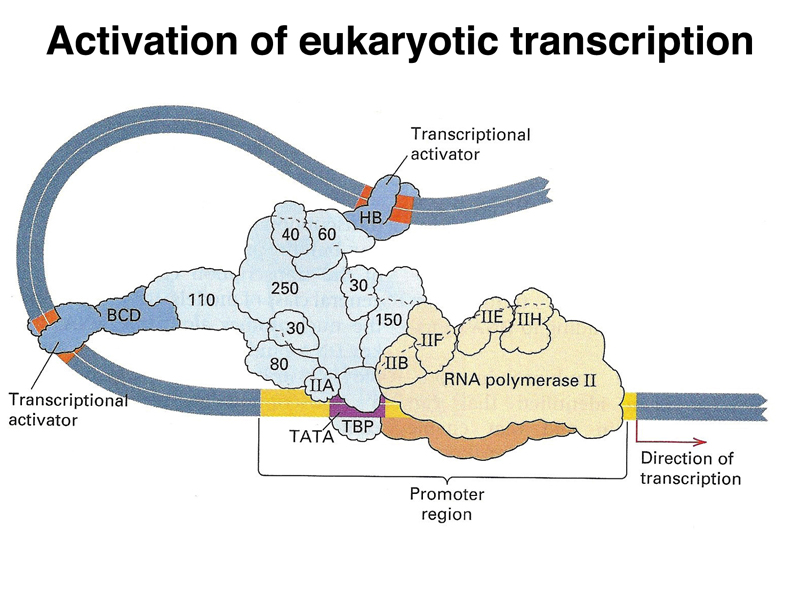
The image below is a cartoon of a eukaryotic transcription activation complex. There are three types of protein components. There is a large collection of proteins that make up a generalized transcription activator (light blue). This complex recruits RNA polymerase (beige) and its cofactors to the promoter region. There are also specific transcription factors (dark blue) that serve as activators. These interact with both the transcription activator complex and specific DNA sequences near the gene, called enhancers. By regulating the expression of these specific transcription factors, an organism can regulate the expression of a large number of genes that are under the control of a particular transcription factor. It is common for a gene to have multiple transcription factors and their enhancers that act in combination, allowing a fine-tuning of the pattern of expression of a gene or set of genes.
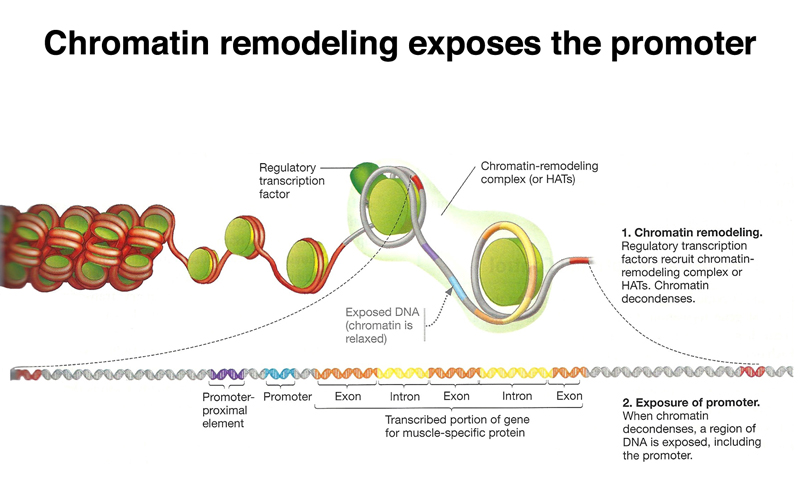
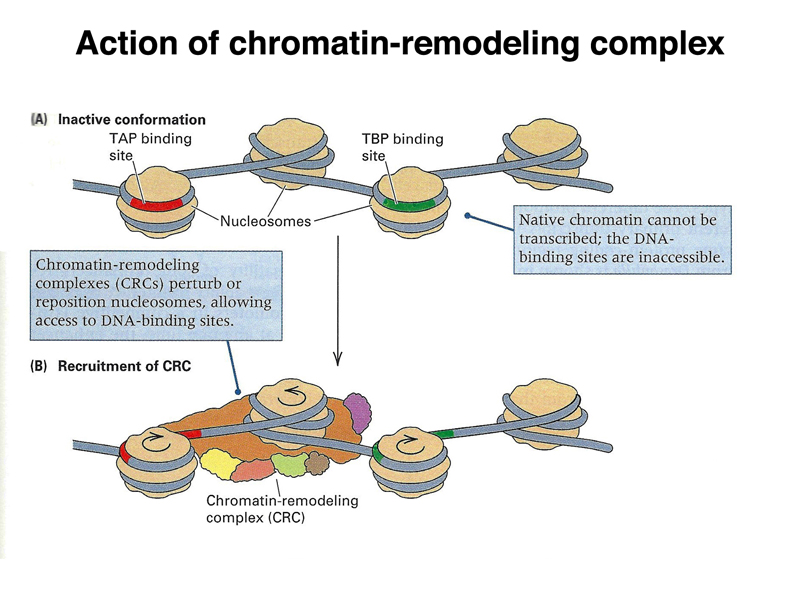
Besides the influence of the overall state of chromatin on accessibility of genes for transcription, in order for a specific gene to be transcribed, its regulatory sequences must be exposed. This is accomplished through chromatin remodeling factors that move regulatory sequences out of nucleosomes, so that they can be recognized by the protein complex that initiates transcription, as shown below.
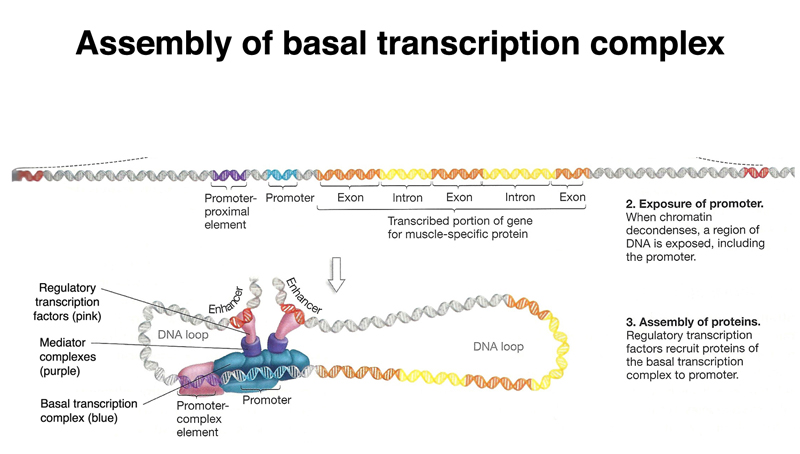
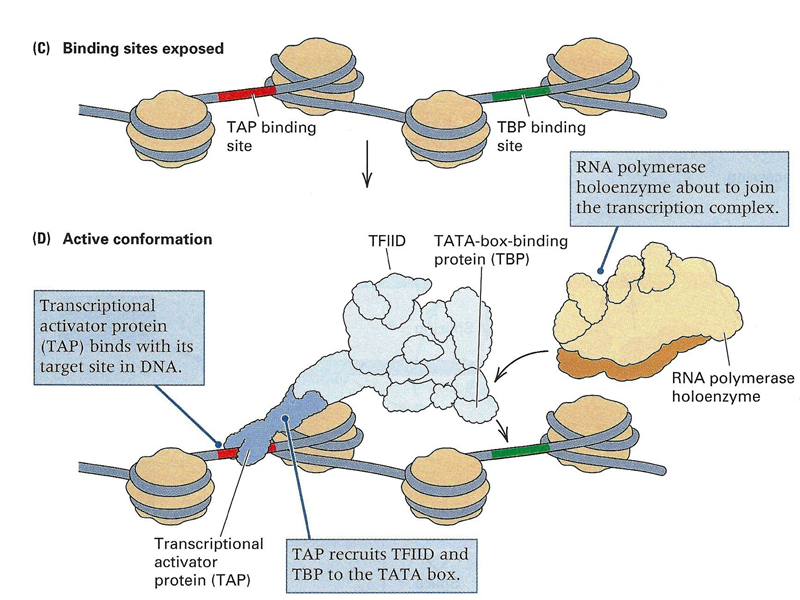
Once regulatory sequences have been exposed, the basal transcription complex assembles over the promoter, while regulatory transcription factors bind enhnacers, as shown below. Note in the drawing below that one of the enhancers is downstream of the gene.
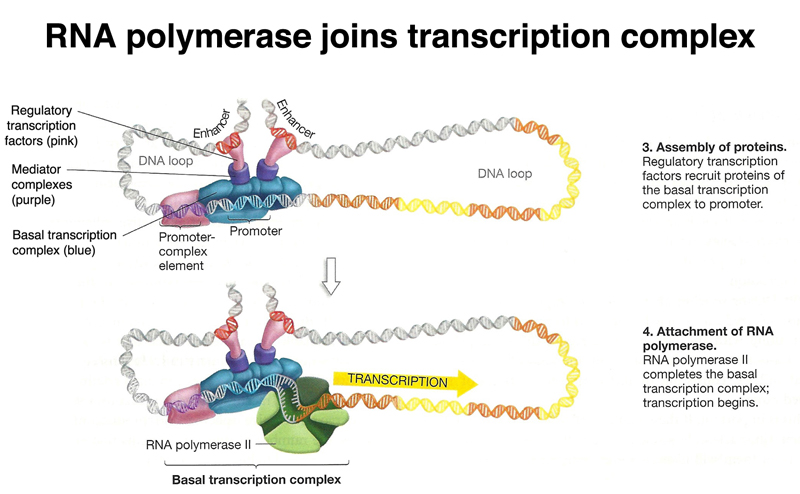
RNA polymerase is then free to join the transcription complex and initiate transcription, as shown below.
The figure below shows another view of the chromatin-remodeling complex, which unwinds DNA from nucleosomes to expose regulatory sites on the DNA.
As shown below, once enhancers are exposed, transcription factors can bind them, and the basal transcription complex can take its position at the promoter.
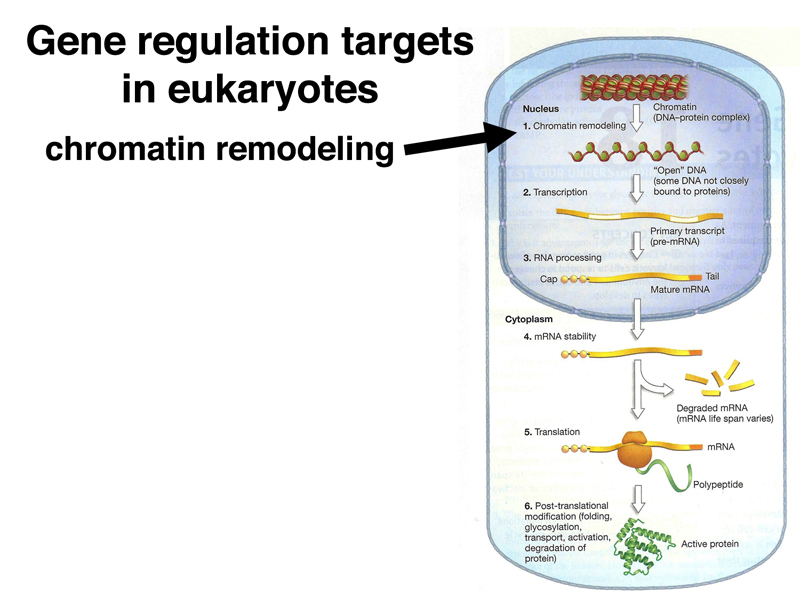
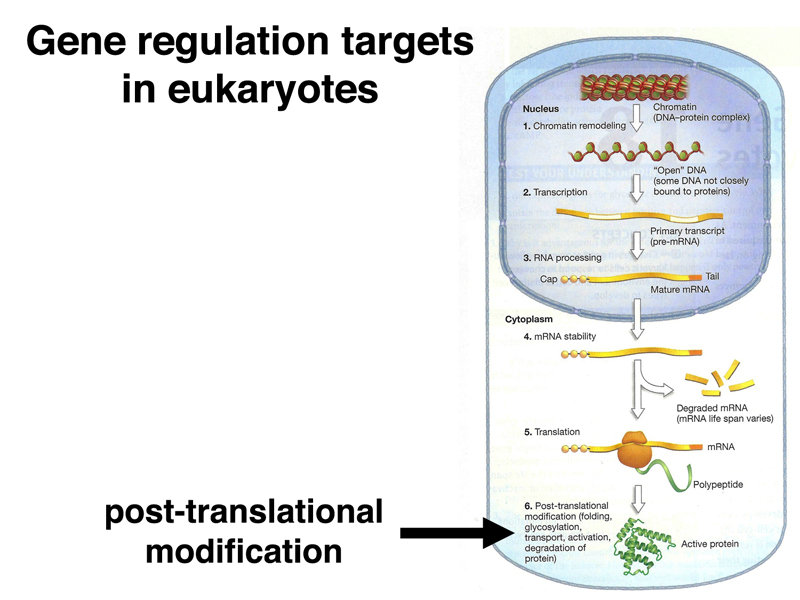
It is useful to consider all the targets of eukaryotic gene regulation that we have encountered to this point. As shown below, chromatin stucture, both the overall chromatin structure and phasing of nucleosomes over particular enhancers, is a target of regulation.
Because there are often many enhancer elements that regulate the expression of a particular gene, the expression or lack of expression of a specific transcription factor often determines whether a particular gene is expressed in a specific tissue at a given time. Most transcription factors have many targets, so controlling the expression of a specific transcription factor can affect the expression of hundreds of genes. This is by far the foremost level of regulation of most eukaryotic genes.
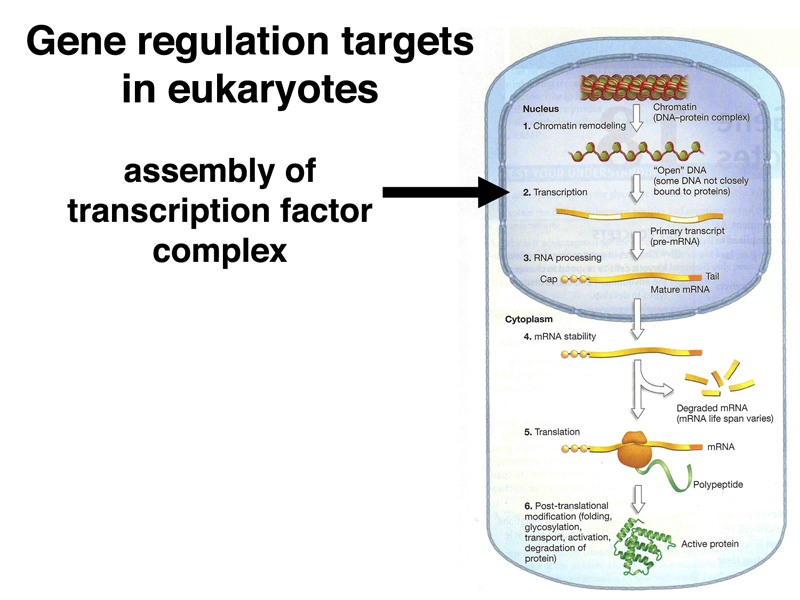
Eukaryotic primary transcripts are processed into mRNA through splicing. As we will see in today's lecture, control of alternative splicing is an important regulatory mechanism in some cases.
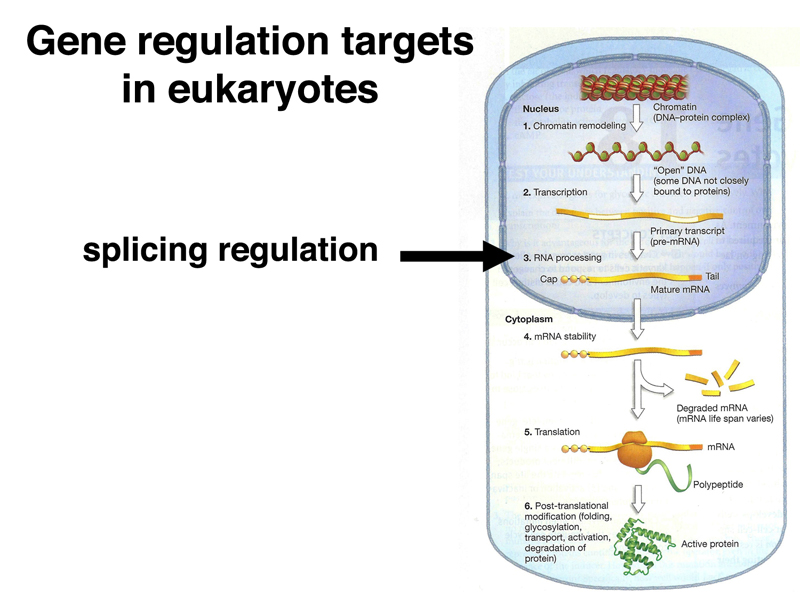
When we reviewed the lac operon, we encountered the phenomenon of nonsense-mediated decay, an example of regulation of mRNA stability in prokarytoes. Nonsense-mediated decay also occurs in eukaryotes. There are many other examples of regulation of gene expression through the regulation of mRNA stability.
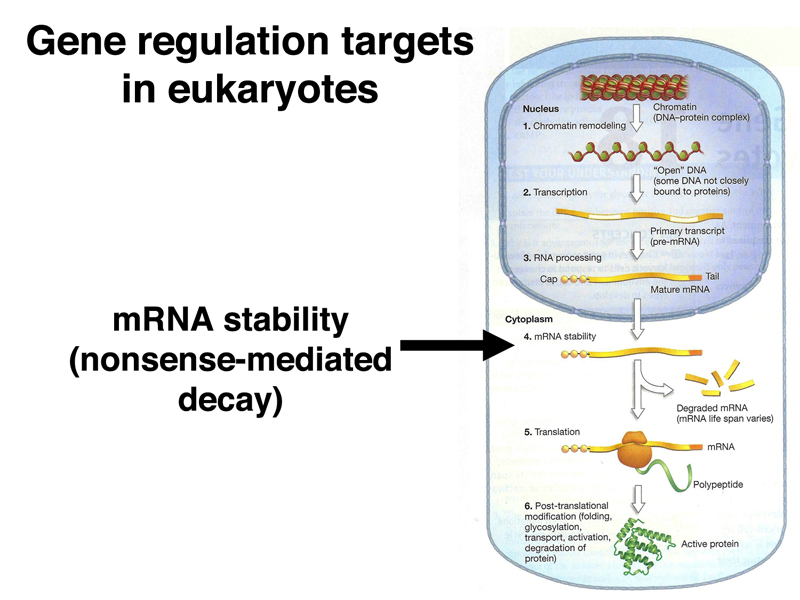
We will also encounter translational control in today's lecture. There are many additional examples of the regulation of gene expression through the regulation of translation.
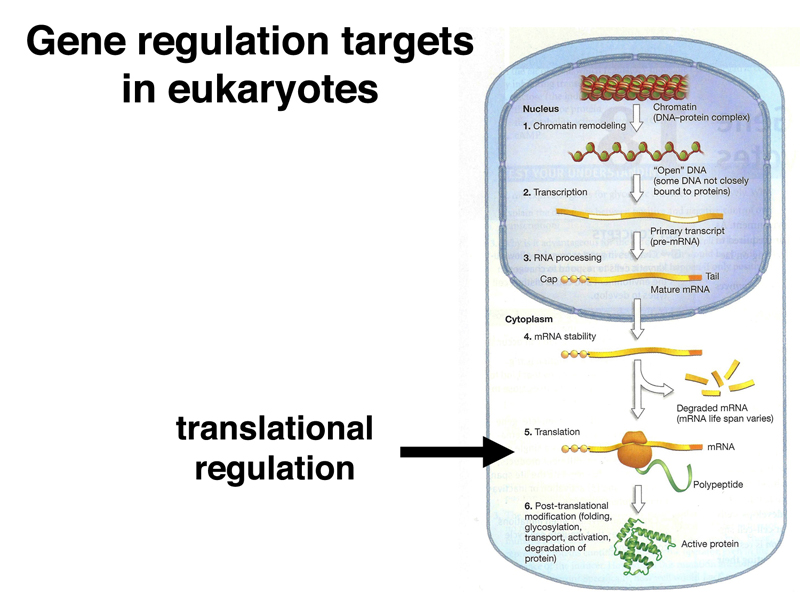
Finally, post-translational modification is an important mechanism for the regulation of gene expression. For example, the post-translational modification of histones, which we have discussed extensively, modifies their activity by increasing or decreasing the accessibility of DNA to other proteins.
In the previous lecture, we discussed male-specific lethal mutations as the key to understanding the mechanism of dosage compensation. The male-specific lethal genes and the function of the proteins that they encode are summarized below.
| Gene | Protein function |
| mle | dsRNA binding |
| msl-1 | chromatin binding |
| msl-2 | chromatin binding (zinc finger) |
| msl-3 | methylated histone binding |
| mof | histone acetyltransferase |
For any of these genes, for example mle, males that are homozygous or hemizygous for loss-of-function alleles die as third-instar larvae. The dying larvae can be shown to have a failure of dosage compensation, that is, they do not make a specific histone modification on the X chromosome, and hence do not hypertranscribe their X chromosome relative to females. Females homozygous for loss-of-function alleles of any of these genes are not affected.
There are also sex-transforming mutations in Drosophila. The sex-transforming mutations and their phenotypes are summarized below.
| Genotype | Phenotype |
| XY; tra/tra | normal male |
| XX; tra/tra | sterile male |
| XY; tra2/tra2 | normal male |
| XX; tra2/tra2 | sterile male |
| XY; dsx/dsx | intersex |
| XX; dsx/dsx | intersex |
| XY; fru/fru | males court but don't mate |
| XX; fru/fru | normal female |
We can ask whether dosage compensation is influenced by sex in Drosophila by constructing double mutants, as shown below.
| Genotype | Phenotype |
| XY; mle/mle | inviable due to failure of dosage compensation |
| XX; mle/mle | normal female |
| XX; mle/mle; tra/tra | sterile male |
Females transformed into sterile males through a mutation in tra are not killed by mle. This shows that the lethality of mle is due to a failure of dosage compensation, and not some other aspect of being male. It also shows that sex itself is not the signal to activate dosage compensation in Drosophila.
Recall that, in humans, Klinefelter males (XXY) have Barr bodies, showing that sex itself is not the signal to activate dosage compensation in humans.
With this in mind, we ask what regulates dosage compensation in Drosophila? We know that it is necessary to have five genes expressed in males in order to activate dosage compensation: mle, msl-1, msl-2, msl-3, and mof. If these genes are necessary and sufficient to initiate dosage compensation, we asked what would happen to females that expressed this collection of genes. The class agreed that this would kill females. So all that is necessary is to identify mutations that kill females only, which has been done.
Recessive loss-of-function alleles of Sex-lethal, such as Sxlf1, kill homozygous females. Dominant gain-of-function alleles, such as SxlM1 kill males, as summarized below.
| Genotype | Phenotype |
| Sxlf1/Y | normal male |
| Sxlf1/Sxlf1 | inviable |
| SxlM1/Y | inviable |
| SxlM1/SxlM1 | normal female |
The male-killing allele SxlM1 kills males even in the presence of a duplication of the wild-type allele of Sxl. The genetics of Sxl were well understood for a long time before we learned the nature of the gene product.
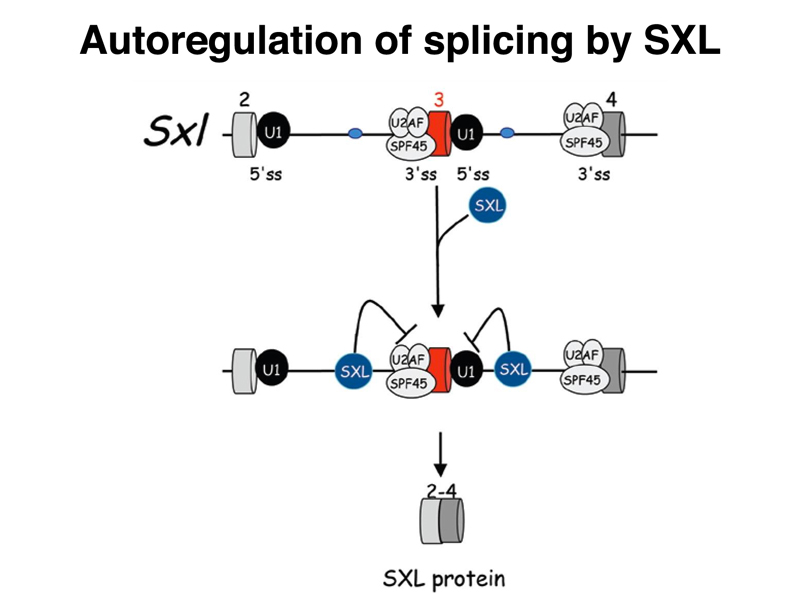
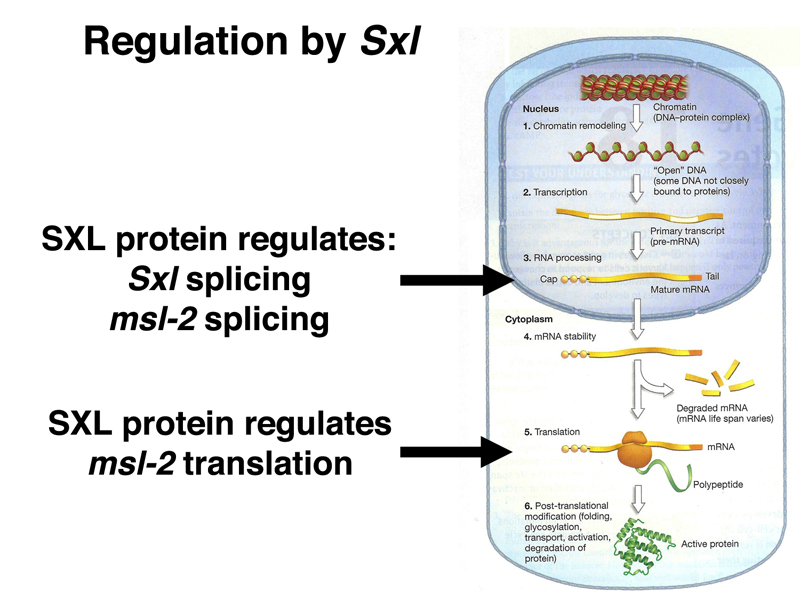
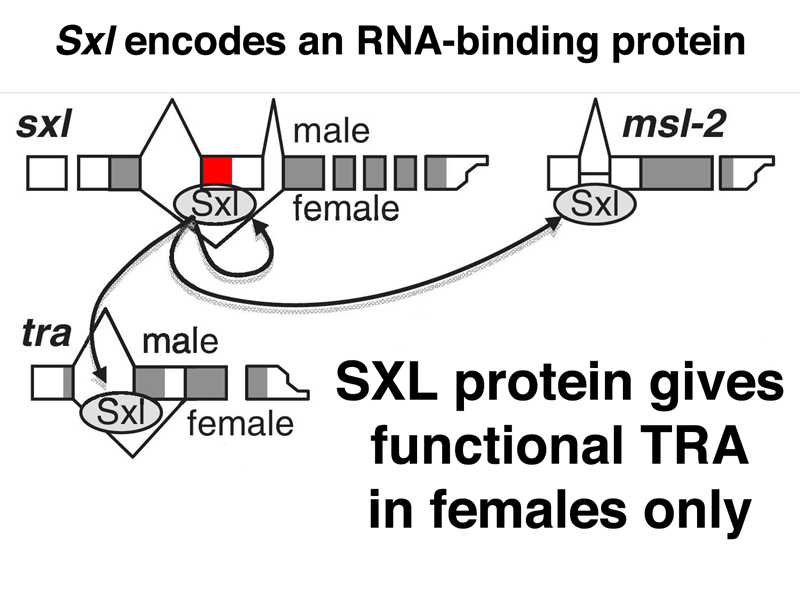
As shown below, Sxl encodes an RNA-binding protein. The SXL protein binds to a number of RNAs, including the primary transcript of Sxl itself. In the absence of SXL protein, the primary transcript is usually spliced to include a toxic exon that contains a stop codon (shown in red below). In the presence of SXL protein, the toxic exon is skipped, and the mRNA encodes functional SXL protein. Therefore, the first target of SXL regulation is Sxl itself, regulated at the level of mRNA processing.
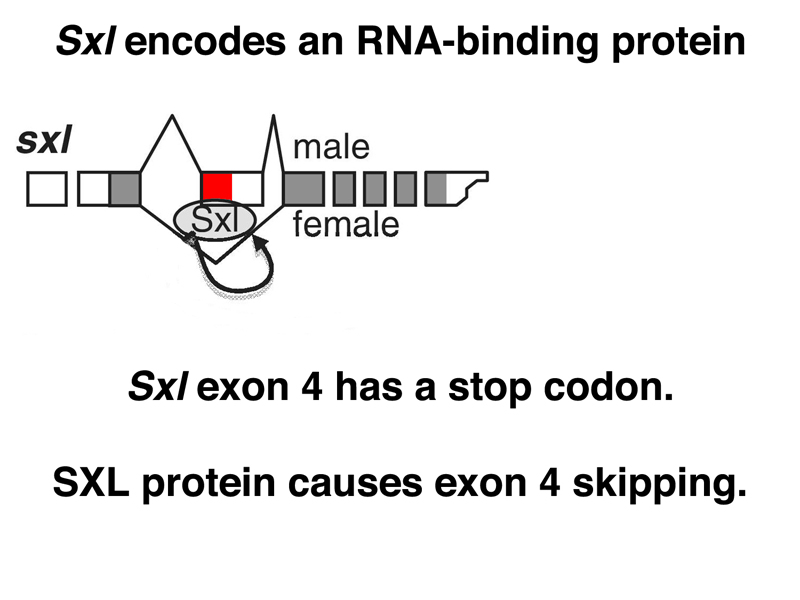
In early embryonic development, there is no dosage compensation. When transcription of the zygotic genome begins, there is an excess of several X-linked transcriptional activators in XX embryos. These positively regulate transcription of Sxl, so that in XX embryos, some functional SXL protein is produced. Sxl autoregulation through the control of splicing results in the expression of SXL in XX embryos but not in XY or X0 embryos. Later in development, expression of Sxl occurs from a different promoter (the maintenance promoter). The effect of transcriptional and splicing regulation is to allow the expression of SXL in XX embryos only.
The figure below shows the interaction of SXL with splicing factors to allow it to skip the toxic exon in females.
All of this would not be of much use if SXL did not have another regulatory target. SXL also regulates the splicing of a male-specific lethal gene that functions in dosage compensation, msl-2, as shown below.
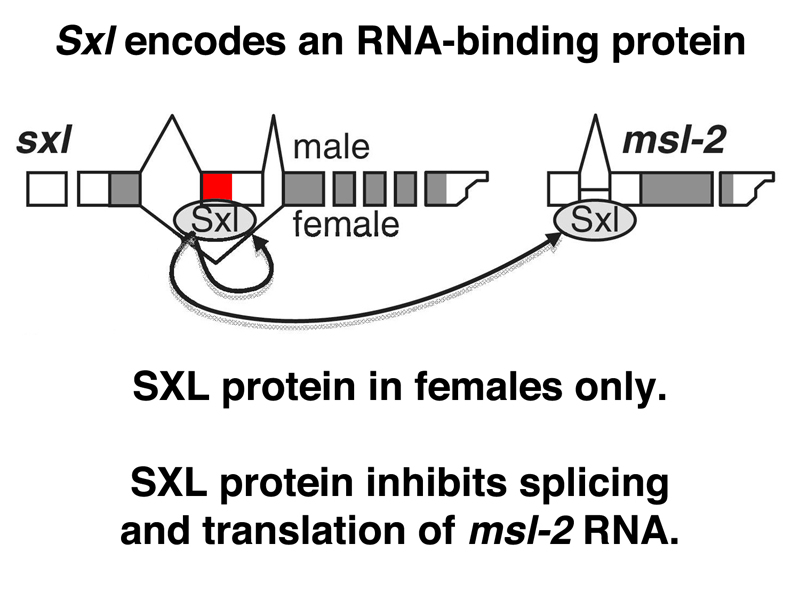
In the absence of SXL protein, the male-specific splice takes place, and there is functional MSL-2 protein. In females, SXL protein interferes with both the splicing and the translation of msl-2 RNA, and there is no MSL-2 protein. Recall that the absence of any of the protein products of the male-specific-lethal genes prevents the complex from associating with the X chromosome and modifying its histones to allow dosage compensation. Surprisingly, all of the other male-specific lethal genes are expressed in females as well. Only msl-2 is the target of regulation that allows the sex-specific activation of dosage compensation.
The figure below shows that SXL protein inhibits translation of msl-2 mRNA by blocking the binding of the small ribosomal subunit to the ribosome-binding site.
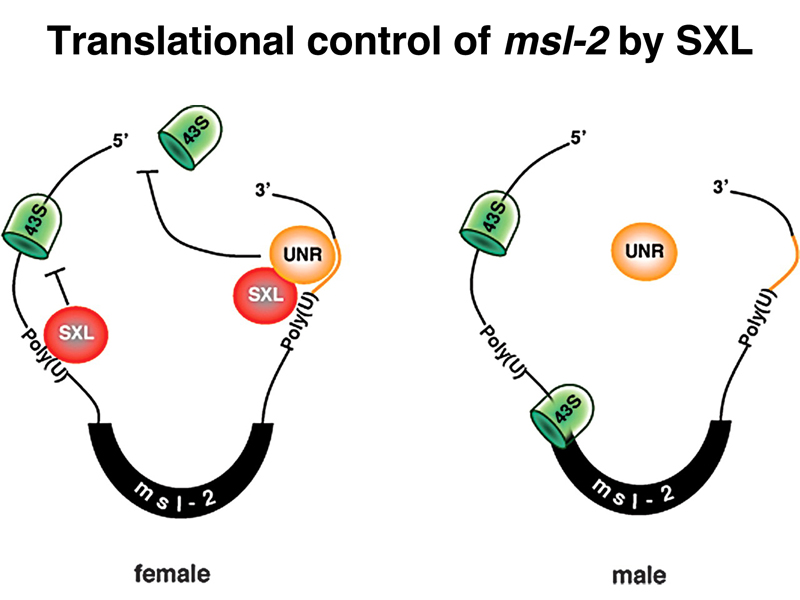
We have now seen three targets of SXL regulation: splicing of Sxl and msl-2 RNAs, and translation of msl-2 mRNA, as summarized below.
At this point, we asked students to consider what the phenotype of a loss-of-function allele of Sxl would be. Because Sxl function is required to negatively regulate dosage compensation in females, in the absence of Sxl function, females would hypertranscribe their X chromosomes and die. Indeed, this is the phenotype by which Sxl was identified.
We also asked students to consider what would be the phenotype of females homozygous for a loss-of-function allele of Sxl and also homozygous for a loss-of-function allele of mle. We expect females homozygous for a loss-of-function allele of Sxl to die of inappropriate dosage compensation, but because a loss-of-function allele of mle would eliminate dosage compensation, these females would be viable.
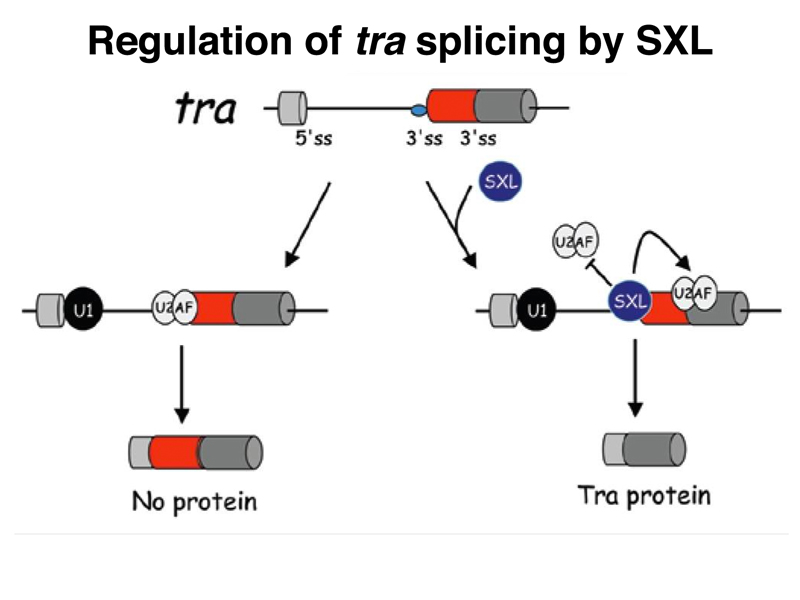
The SXL protein binds to another RNA besides Sxl and msl-2 RNAs, the RNA for the sex-transforming gene tra, as shown below. In the presence of SXL, the tra transcript is spliced to make a functional TRA protein. In the absence of this female-specific splice, the default splice produces an mRNA with no long open reading frame, and hence no functional TRA protein. Recall that we saw earlier that recessive alleles of tra only affect females, transforming them into sterile males. This makes sense, now that we know that males do not express the TRA protein.
Control of tra splicing by SXL is shown in more detail in the figure below.
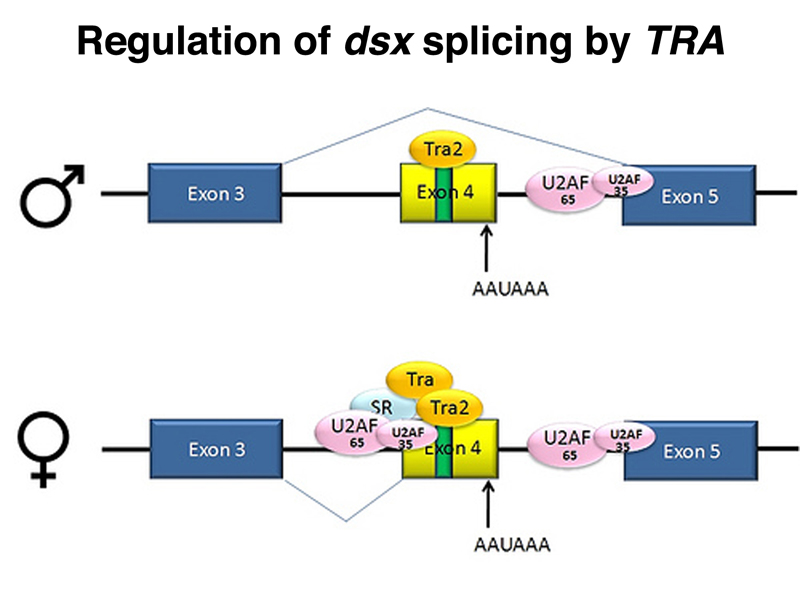
The female-specific TRA protein is also an RNA-binding protein that regulates the splicing of two different RNAs, as shown below. In the presence of TRA, the dsx RNA is spliced to express a female-specific form of DSX, DSXF. In the absence of TRA, the dsx RNA is spliced to express a male-specific form of DSX, DSXM. In the presence of TRA, the fru RNA is spliced to produce an inactive form specific to females, while in the absence of SXL, the fru RNA is spliced to produce the active form specific to males.
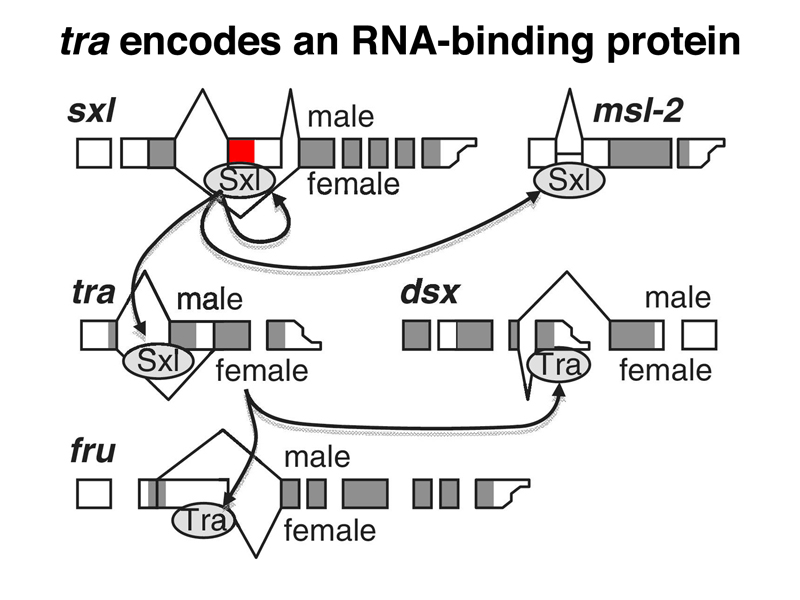
The sex-specific splicing of dsx under the control of TRA is shown in more detail in the figure below.
We summarize the targets of regulation by TRA in the image below.
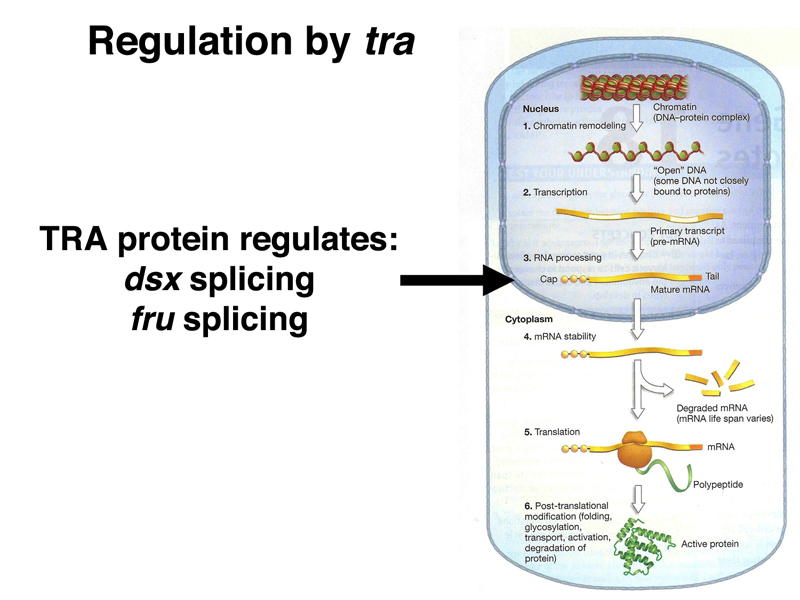
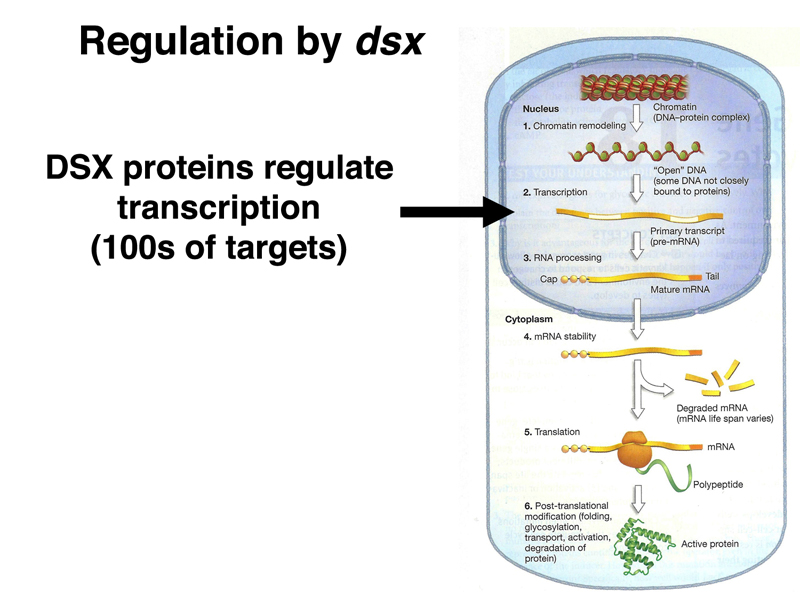
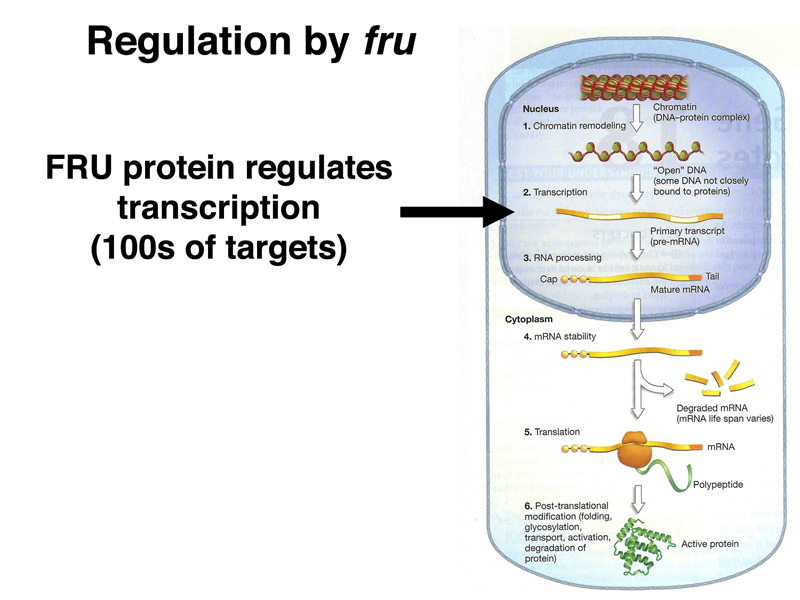
Both dsx and fru encode transcription factors with many target genes. The DSXF and DSXM transcription factors promote female and male somatic sex, respectively (remember that loss of dsx function changes both males and females into intersexes).
The images below summarize the targets of regulation by DSXF, DSXM, and FRU.
The regulatory gene Sxl connects the processes of sex determination and dosage compensation, as summarized in the figure below.
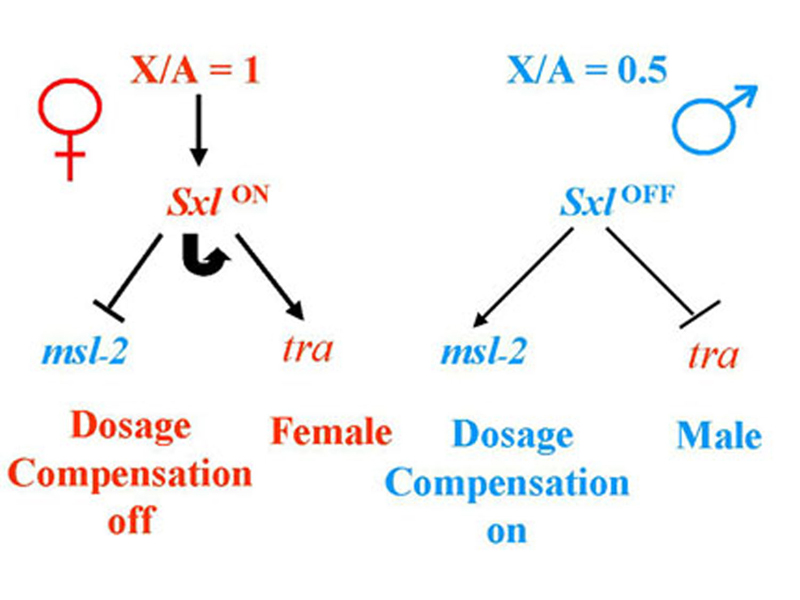
In the presence of two X chromosomes (not an X:A ratio of 1 as shown in the figure), Sxl is on, and there is functional SXL protein. SXL protein inhibits dosage compensation through negative regulation of splicing and translation of msl-2, and directs female development by regulating the splicing of tra RNA to produce functional TRA protein in females only.
In the presence of one X chromosome (not an X:A ratio of 0.5 as shown in the figure), Sxl is off, and there is no functional SXL protein. The msl-2 RNA is spliced and translated to produce functional MSL-2. Because all of the other male-specific lethal genes are expressed constitutively, dosage compensation is activated. In the absence of SXL, there is no functional TRA, and male development occurs.
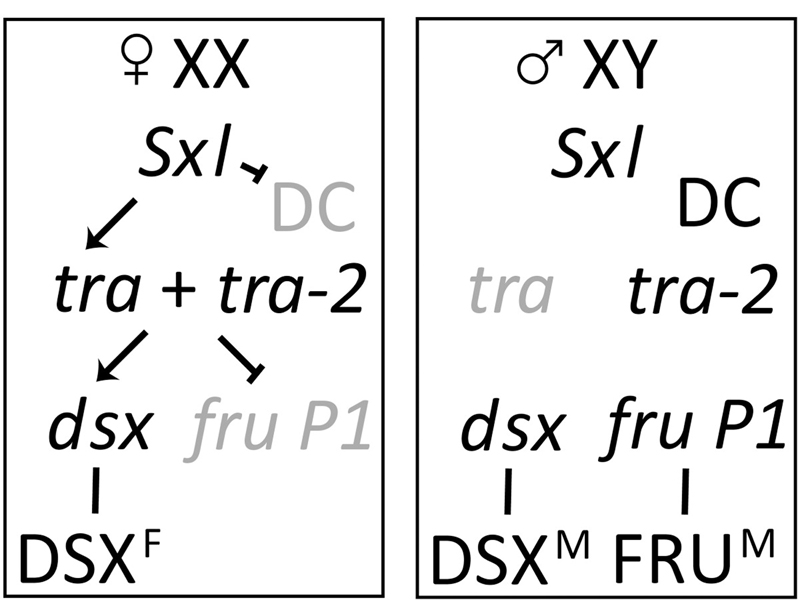
The image below adds in the targets of TRA: dsx and fru. In the presence of TRA, dsx RNA is spliced to encode DSXF, while fru RNA is spliced to produce a nonfunctional product. In the absence of TRA, dsx RNA is spliced to encode DSXM, and fru is spliced to encode a functional FRU protein.
We have now seen examples of almost every level of gene regulation in our discussion of sex determination and dosage compensation in Drosophila, as summarized below.
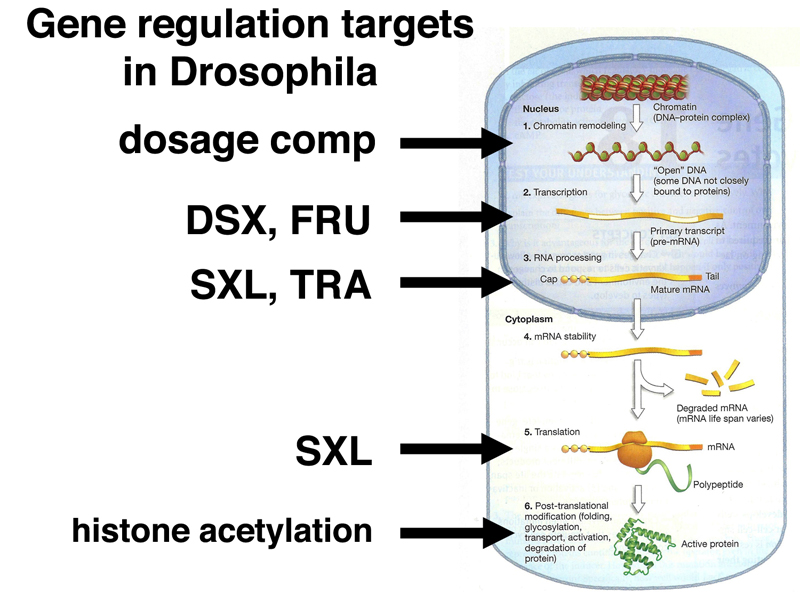
The process of dosage compensation regulates X chromosome transcription in males through a change in the chromatin state. DSXF, DSXM, and FRU are transcription factors that regulate the initiation of transcription at hundreds of target genes. Both SXL and TRA are RNA-binding proteins that regulate alternative splicing of RNA during the production of mRNAs. SXL also inhibits translation of the mRNA for msl-2. Finally, we have discussed post-translational modification of histones as a way of affecting their activity.