
| Home | Syllabus | Schedule | Lecture Notes | Extras | Glossary |

| Home | Syllabus | Schedule | Lecture Notes | Extras | Glossary |
We have adapted this approach to presenting DNA structure from that used by Eric Lander in his lectures at MIT.
We began by reviewing the story of heredity as we developed it over the previous lectures. Mendel showed us that heredity was controlled by particles called genes that are not affected by their passage through hybrids. Flemming and the cytologists that followed after him described the behavior of chromosomes in mitosis and meiosis. Sturtevant, working in Morgan's lab, showed through the construction of linkage maps that genes were organized into linear arrays, strongly suggesting that genes are carried on chromosomes. Bridges, working in Morgan's lab, showed that genes are on chromosomes by demonstrating that abnormal inheritance of X-linked genes in Drosophila is correlated with abnormal inheritance of chromosomes.
There is another discovery that we have not discussed in detail that begins another thread to this story. Garrod described four inborn errors of metabolism (alkaptonuria and albinism among them), showing that genes could control the activity of enzymes. It is very interesting that so little is made of Garrod's insight in 1908, and for decades after.
If a variant allele of a gene causes its phenotype by making a nonfunctional protein (as is the case in albinism), perhaps it is the function of genes, whatever they are, to direct biochemistry. This makes us ask the question, what is the biochemical basis of heredity?
Biochemistry is about fractionating and purifying biomolecules from cells, and is typically based on having an assay. For example, we know that hemophilia results from an inherited deficiency in a clotting factor. We could take blood from normal people and fractionate it, looking for a molecule that restored clotting ability to blood from hemophiliacs. This approach worked well, and for a period of time hemophiliacs were treated with clotting factor concentrates from hundreds of blood donors. The AIDS epidemic began when this practice was in use, and many hemophiliacs eventually died of HIV infections before a reliable test to screen blood for HIV was available.
If we treat a hemophiliac with clotting factor, their phenotype is normal, but they will still transmit the variant allele causing hemophilia to their children. So while clotting factor concentrate is an effective therapy, the purified protein is not a molecule that governs heredity. What made people think that they could ever purify heredity in a test tube?
We stepped back in time to the early part of the nineteenth century. In 1828, Wöhler accidentally synthesized urea. It was the first time that a compound produced by living things had ever been chemically synthesized. It was universally believed at the time that organic compounds could only be made by living things. Once Wöhler had disproven this, thousands of organic compounds were synthesized, yet the field of the chemistry of carbon compounds is still called organic chemistry.
In 1944, the physicist Erwin Shrödinger published a book called "What is Life?" based on a series of lectures he had given. He posed the interesting question whether everything that happened in living things could be explained by physics and chemistry. He speculated about the nature of heredity. His work stimulated many people from outside the field of biology to take up the question of the chemical nature of the gene.
In 1918, the world was hit by the worst influenza outbreak in history. The 1918 Spanish flu infected 500 million people worldwide, killing 50 - 100 million people, about 3 - 5% of the world's population. Part of the response to this public health crisis was the appointment of Frederick Griffith to head a small laboratory in London to study means of protection against such a pandemic.
It was known that the major cause of death among people infected with the Spanish flu was pneumonia caused by Streptococcus pneumoniae, aka pneumococcus. Griffith had isolated and serotyped many strains of pneumococcus. There are both virulent and nonvirulent strains. One nonvirulent strain is distinguished by its appearance when grown on culture plates: it is "rough" as opposed to the "smooth" appearance of colonies of a virulent strain. The difference between the two strains is that smooth virulent strains make a polysaccharide coating that protects them from the host immune system. This polysaccharide coat produces the glistening smooth appearance of the colonies when the virulent strain is grown on culture plates.
Griffith conducted a set of experiments injecting mice with pneumococcus, summarized in the drawing below.
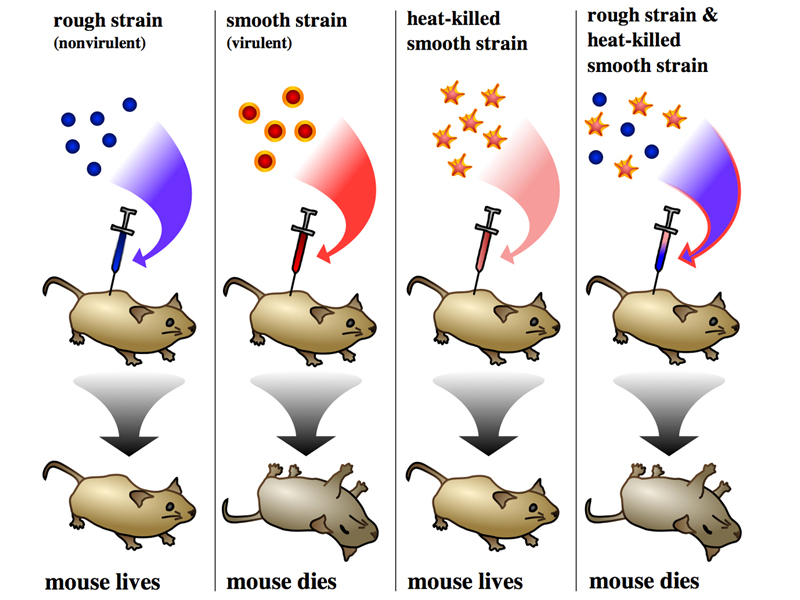
As shown in the first panel, mice injected with the living rough nonvirulent strain survive. As shown in the second panel, mice injected with the living smooth virulent strain die. Living smooth pneumococcus can be recovered from the blood of these mice. In the third panel, we see that when the smooth virulent strain is heat-killed before injection, the mouse survives. This shows that the virulent strain must be alive to kill the mouse, so the mouse does not die from some toxin present in the smooth strain, it dies of an infection by living bacteria.
The last panel shows a surprising result, so strange that it is difficult to understand exactly what Griffith was thinking when he conducted this experiment. If living rough nonvirulent pneumococcus (which doesn't kill mice) is mixed with heat-killed smooth virulent pneumococcus (which doesn't kill mice), the combination kills mice. Moreover, smooth virulent pneumococcus can be recovered from these mice. Because some unknown substance in the heat-killed smooth cells can transform rough nonvirulent bacteria to a virulent strain, this experiment demonstrates the existence of a transforming principle. But what was the transforming principle?
Griffith began experiments to fractionate the heat-killed smooth strain to isolate the molecule that was the transforming principle. Unfortunately, in 1941, Griffith was killed by a German bomb during the Blitz of London.
A group of other researchers, Oswald Avery, Colin MacLeod, and Maclyn McCarty, took up Griffith's work at Rockefeller University shortly thereafter. Their principal innovation was to realize that because mice injected with the combination of heat-killed virulent smooth pneumococcus and living nonvirulent rough pneumococcus die of an infection by transformed pneumococcus (which appears smooth when cultured), perhaps a better assay for transformation is the change in the appearance of the colonies. They eliminated the injection of mixtures into mice, opting instead for watching for the transformation of rough colonies to smooth colonies by various fractions of the heat-killed smooth strain.
They quickly identified the tranforming principle as the nucleic acid DNA. Fractions consisting of protein, RNA, carbohydrate, or lipid did not have tranforming activity. Avery was very reserved at first about his findings, delaying publication for a considerable time until finally revealing his results to the scientific community. The results were very poorly received. No one believed that DNA would be the genetic material, because it was thought to be a very boring molecule that was a structural component of chromosomes, serving only to hold the protein molecules that made up genes in their place.
It was very difficult to argue against the notion that a minute quantity of a vital protein component was purifying along with the DNA, so the results convinced very few scientists. It is easier to understand this if we review what was known about DNA at the time.
DNA was first isolated by Friedrich Miescher in 1869. It was known to have three main chemical components: a carbohydrate (deoxyribose), phosphate, and four different nitrogenous bases, adenine, thymine, guanine, and cytosine. The building block of DNA is the nucleotide, a molecule that contains each of these three components.
First, let us consider the carbohydrate, a five-carbon sugar called deoxyribose, shown below.
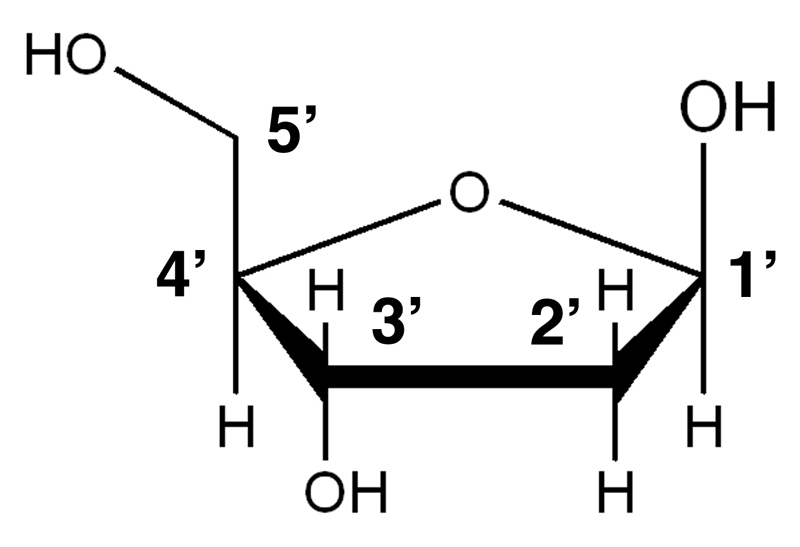
The carbon atoms in the sugar are numbered 1' through 5'. The 2' atom of deoxyribose lacks a hydroxyl group. Adding a hydroxyl group below the plane of the ring in the 2' position would make this ribose, the sugar found in RNA.
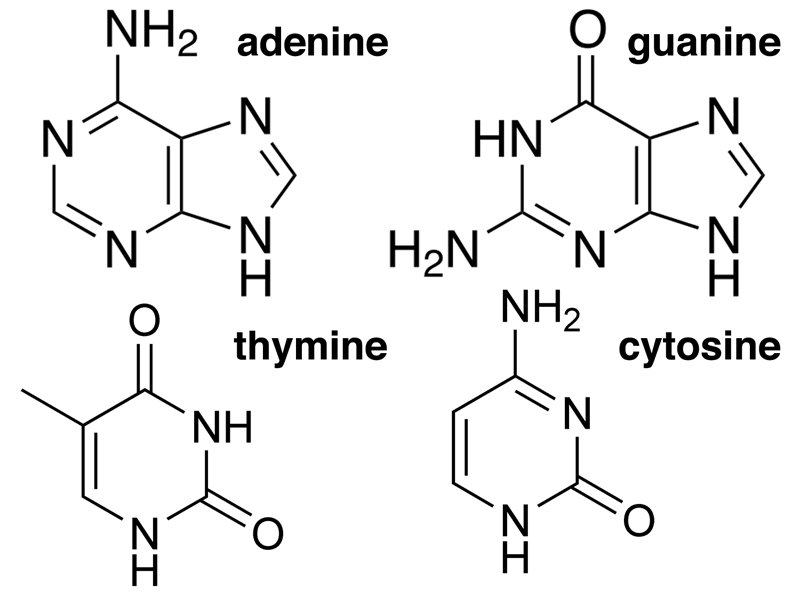
DNA also contains four nitrogenous bases, shown below. The bases are of two types: purines and pyrimidines. Purines are compounds with both a six-member and a five-member ring. The purines found in DNA are adenine and guanine. Pyrimidines are compounds with a six-member ring. The pyrimidines found in DNA are thymine and cytosine. If you need a trick to remember purines and pyrimidines, remember that the larger molecules (purines) have the shorter name, while the smaller molecules (pyrimidines) have the longer name.
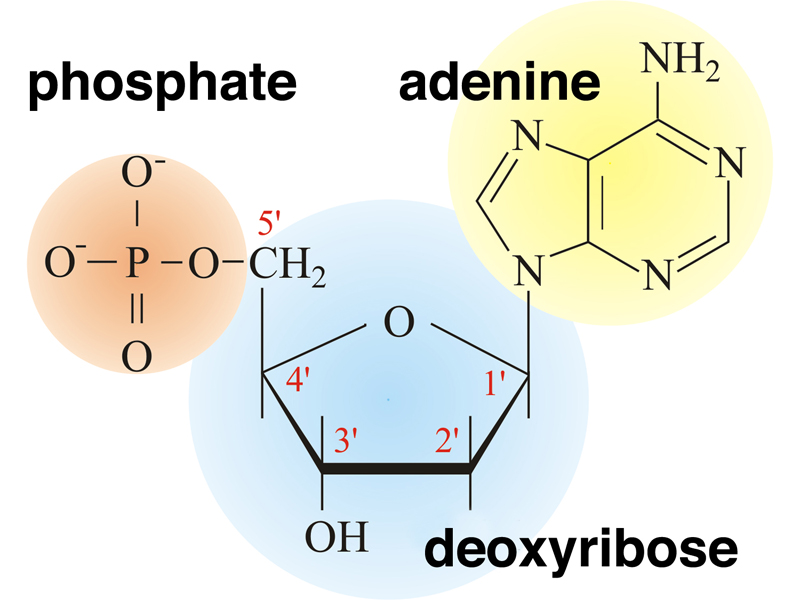
The basic unit of DNA is the nucleotide (actually the deoxyribonucleotide), shown below. The nucleotide has three components: the deoxyribose sugar, a base (in this case adenine) in a glycosidic bond to the 1' carbon of the sugar, and one or more phosphate groups in a phosphodiester bond to the 5' carbon of the sugar. A nucleotide that is ready to be incorporated into DNA has three phosphate groups, and cleavage of the high-energy phosphate bond to leave a single phosphate on the 5' carbon provides energy for DNA synthesis.
You should already be familiar with nucleotides from prior study of biology. If we added a hydroxyl group below the plane of the ring to the 2' carbon, this would change the sugar in the drawing to ribose. If we added two more phosphate groups to the drawing, this nucleotide would be a ribonucleoside triphosphate with adenine as the base. You know this molecule as ATP.
The primary structure of DNA was known in 1944. DNA consists of a sugar-phosphate backbone with phosphodiester bonds between the 5' carbon of one nucleotide and the 3' carbon of the next nucleotide, as shown below.
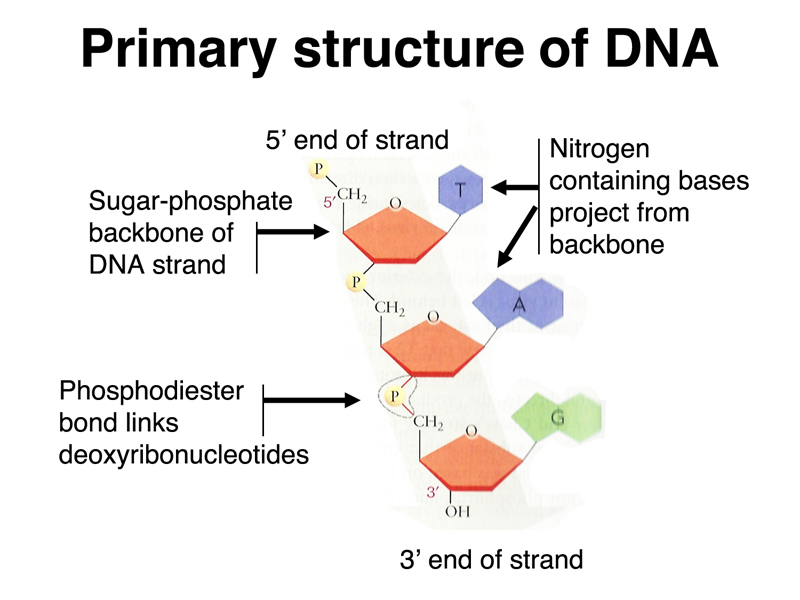
This means that a strand of DNA has a polarity. The 5' end has a single phosphate attached to the 5' carbon at one end of the strand. The 3' end has a hydroxyl group attached to the 3' carbon at the other end of the strand.
To biologists of 1944, DNA was a very boring molecule. The four bases have very similar chemical properties, in contrast to the great diversity of chemical properties found in the R groups of the twenty primary amino acids. We have speculated that because every complex mechanical device in use in 1944 (like the German Enigma encryption machine that we showed) was analog, the idea that a simple molecule could encode complex proteins using a digital code was unfamiliar. Today, we are awash in digital information, ultimately encoded as ones and zeros in computer memory, so the idea of encoding complex information using a simple code is very familiar.
In 1952, eight years after the poor reception of Avery, MacLeod and McCarty's work showing that the transforming principle was DNA, Alfred Hershey and Martha Chase conducted a beautiful experiment at Cold Spring Harbor Laboratory. They were working with the bacteriophage T2. A bacteriophage is a virus that infects bacteria, in this case E. coli. A drawing of bacteriphage T2 is shown below.
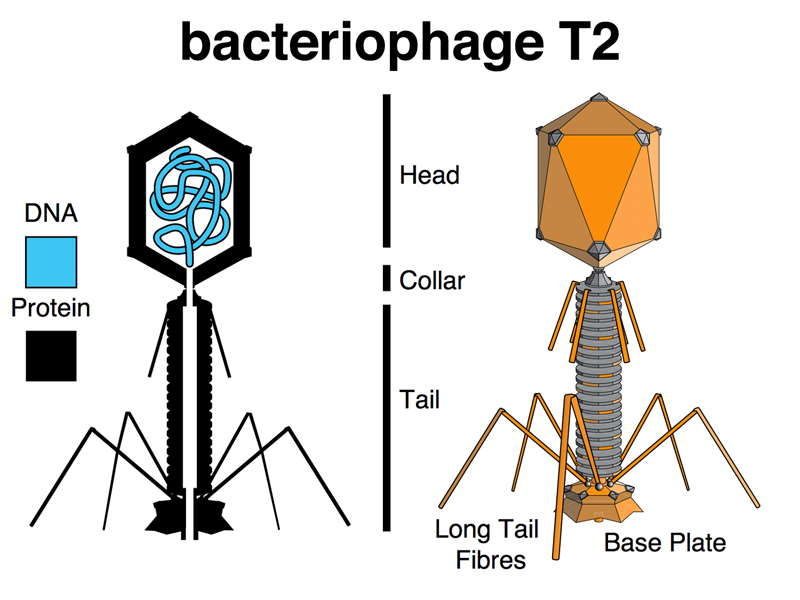
A bacteriophage is a wonderfully simple thing, consisting of a complex assembly of proteins around a headful of DNA. When a T2 particle attaches to an E. coli cell, it somehow transmits information to the bacterial cell that cause the cell to become a factory for making bacteriophage. After about 30 minutes, the bacterial cell bursts open, releasing hundreds of complete phage particles that can infect other bacterial cells. The information that the phage transmits to the bacterial cell can be thought of as heredity, in the same way that the seed from an orange contains all of the instructions to grow an orange tree to make more oranges. We discussed this briefly in class until everyone agreed that the phage's instructions to make more phage was an example of genetic information.
We thought about how we might determine whether DNA or protein was the genetic material. There is no RNA, carbohydrate, or lipid in a T2 phage particle. One student suggested fractionating T2 into DNA and protein and testing each fraction for its effects on E. coli. This is a good idea, but it doesn't work: neither fraction causes E. coli to make phage. Apparently, the phage particle is a machine that needs to be left intact in order to infect E. coli.
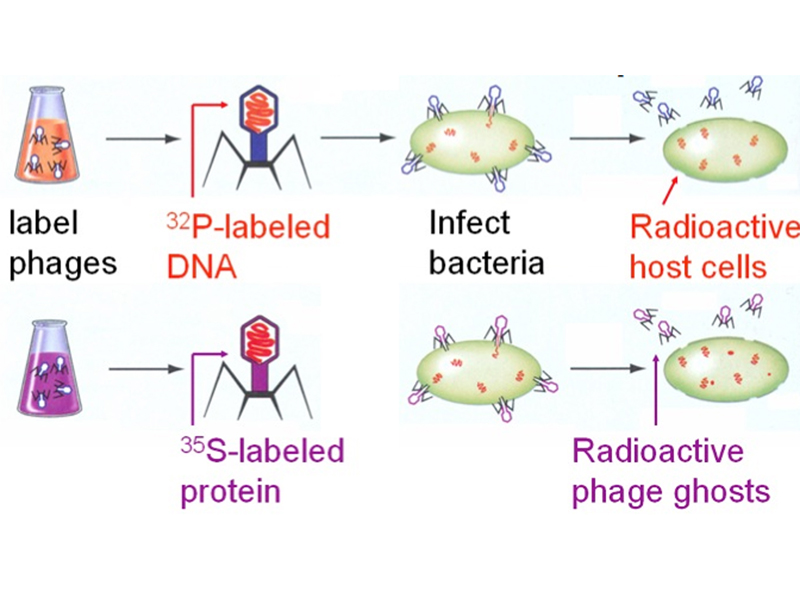
Several other students suggested the approach that was ultimately used by Hershey and Chase: labeling two batches of phage with radioisotopes to follow the DNA and protein. This is actually quite simple. DNA, as we have seen, contains a lot of phosphate. Phosphorus is absent from proteins (some proteins are phosphorylated after they are synthesized, but there aren't any such proteins in phage). Sulfur is absent from DNA, but present in proteins (methionine and cysteine are sulfur-containing amino acids). We can grow bacteria and phage in medium containing inorganic phosphate labeled with 32P to produce phage whose DNA is labeled, or in medium containing inorganic sulfate labeled with 35S to produce phage whose proteins are labeled.
The last thing we need to carry out this experiment is a way to knock the phage off of the E. coli cells once they have had a few minutes to transmit genetic information to the cells. A student suggested centrifuging the culture, but this will pellet E. coli cells with the phages still attached. Another student suggested denaturing agents (like detergent or urea), but these will damage the E. coli cells. The solution used by Hershey and Chase was hydrodynamic shear. They used a scientific instrument capable of delivering just the right amount of hydrodynamic shear to knock the phages off the E. coli cells: a Waring kitchen blender.
They infected E. coli cells with either 32P-labeled phage or 35S-labeled phage, allowed a few minutes for phage adsorption and the transfer of genetic information, then spun the cultures in a blender before pelleting the bacteria in a centrifuge. The phage that were knocked off the bacteria remained in the supernatant.
Their results are shown in the illustrations below. Bacterial pellets are labeled by 32P, while the 35S remains in the supernatant. This shows that the transfer of genetic information from the phage to the bacteria occurs by the transfer of DNA, not protein, demonstrating that the genetic material is DNA.
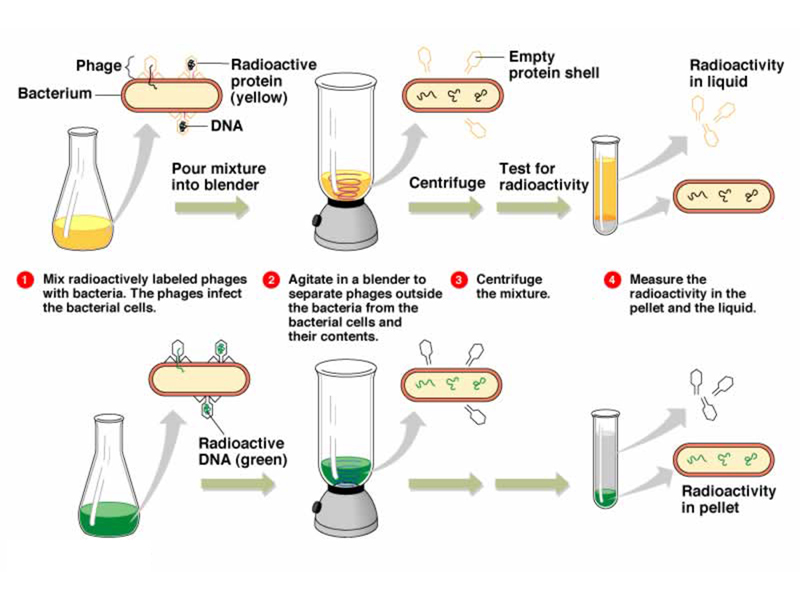
This experiment was accepted by the scientific community in 1952 as proof that genes are made of DNA.
Also in 1952, Austrian chemist Erwin Chargaff determined the amount of A, T, C, and G in DNA from various sources. He found that the quantity of purines (A and G) was always equal to the amount of pyrimidines (C and T). More specifically, he found that the amount of A was always equal to the amount of T, and that the amount of C was always equal to the amount of G, even though the overall percentage of A and T (or C and G) varied in DNA from different sources. Some of his results are shown below.
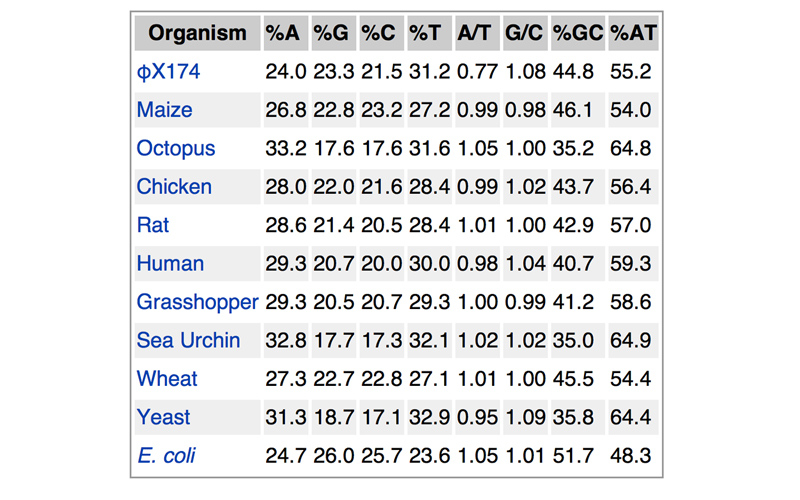
Note that the only organism that breaks the rule that A/T = 1 is φX174, a bacteriophage that is known to carry single-stranded DNA.
The technique that allowed the determination of the secondary structure of DNA is X-ray diffraction. In this method, a crystal of a macromolecule is illuminated with a beam of X rays. The spacings of components of the molecule are on the order of the wavelength of X rays, so the X-ray beam diffracts. The diffraction pattern is captured by the exposure of X ray film.
The first results on DNA were obtained by Astbury in 1933. The easiest thing to see, even on DNA samples that were not very well ordered, was that there was a repeating sturcture with a spacing of 3.4 Angstroms. Astbury correctly interpreted this to be base stacking, where the flat bases are present in the DNA structure like a stack of pennies, with 3.4 Angstroms between the bases.
In 1951, Rosalind Franklin began work on X-ray crystallography at King's College London. She is credited with having taken a single X-ray diffraction image of the B form of DNA that allowed the determination of the secondary structure of DNA. The determination of the structure of DNA has been called the most important biological discovery of the 20th century, making the photograph the most important piece of biological data in the entire 20th century. The image, named by Franklin "Photo 51," is shown below.
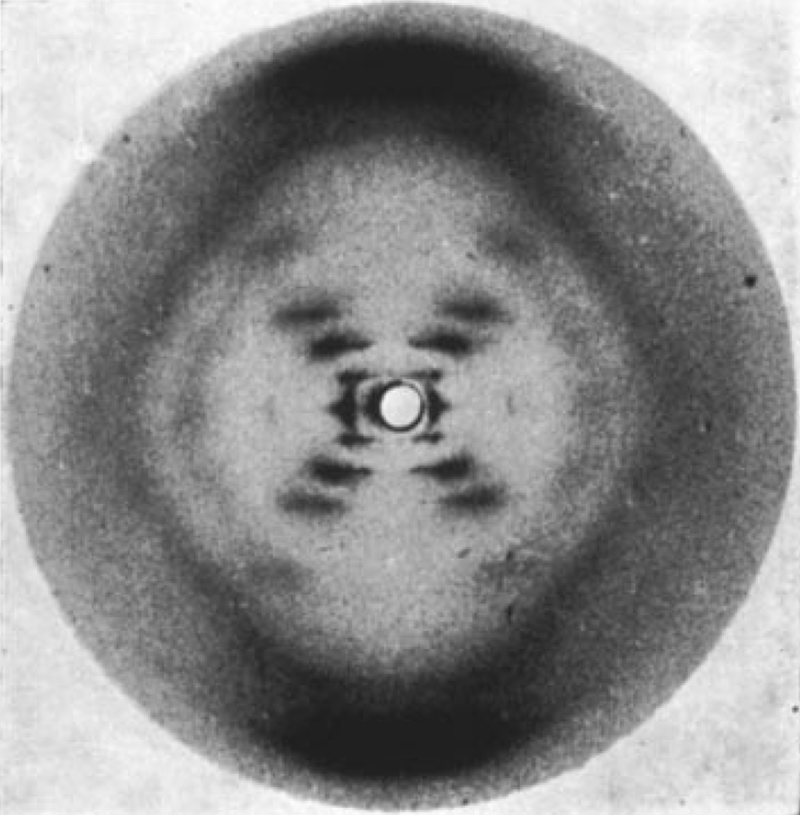
The interpretation of this image is detailed below. I am not a crytallographer and am not able to interpret this image myself. I am presenting this material to show what kinds of information can be extracted from this image. Most of this material is adapted from the presentation to accompany the NOVA program The Secret of Photo 51. You can see the full 53 minute documentary, The Secret of Photo 51, on YouTube.
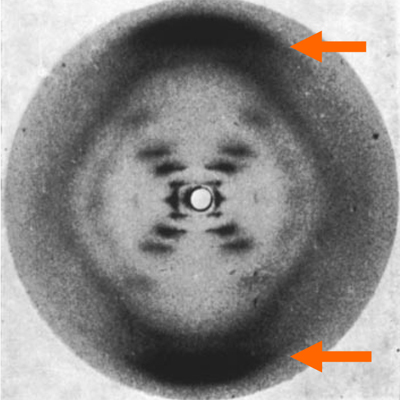 |
The broad smears at the top and bottom of the image show the 3.4 Angstrom spacing of the stacked bases. The smallest spacings cause the beam to diffract the greatest distance. |
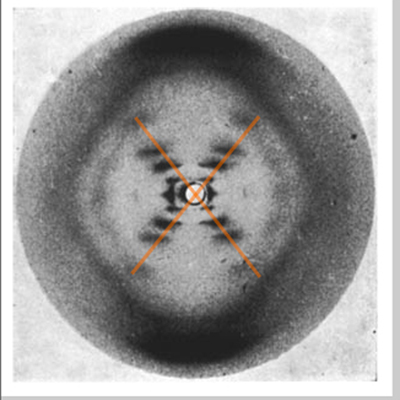 |
The X shape indicates a helix. Franklin's earlier photos of another form of DNA also clearly showed the X indicating a helix, so all early work to solve the secondary structure of DNA incorporated this finding. |
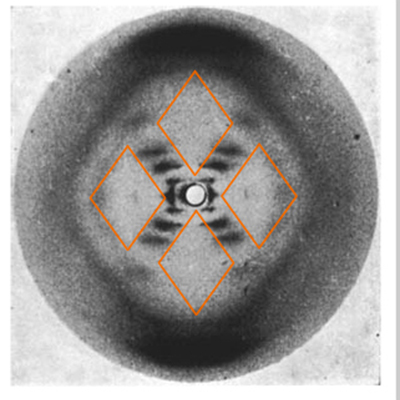 |
The diamonds show that the helix repeats and that many DNA molecules that are not precisely aligned are being imaged. The clear diamonds at the top and bottom, and the murky edges of the diamonds to the left and right, show that the sugar-phosphate backbone is on the outside. |
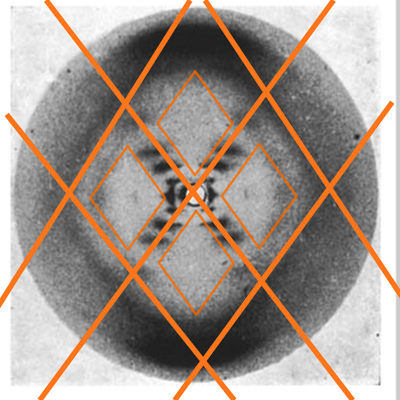 |
The diamonds show that the helix repeats and that many DNA molecules that are not precisely aligned are being imaged. |
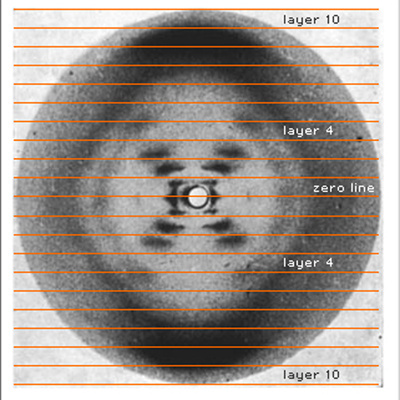 |
Smears organized along layer lines show that there is a repeating helical structure. |
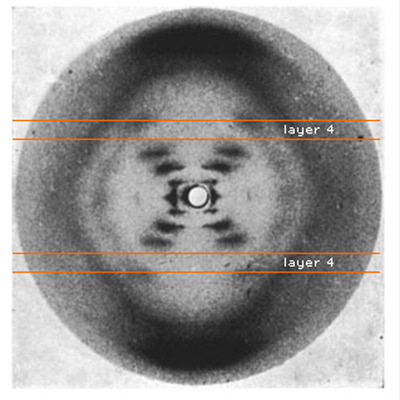 |
The absence of a smear at layer line 4 shows where the two strands of the helix cross. |
The image below shows how X-ray diffraction data can be used to determine three different measurements: the tilt of the helix, the distance between bases, and the distance for one complete turn of the helix. Note also in the model to the right that you can see points at which the two strands of the helix cross.
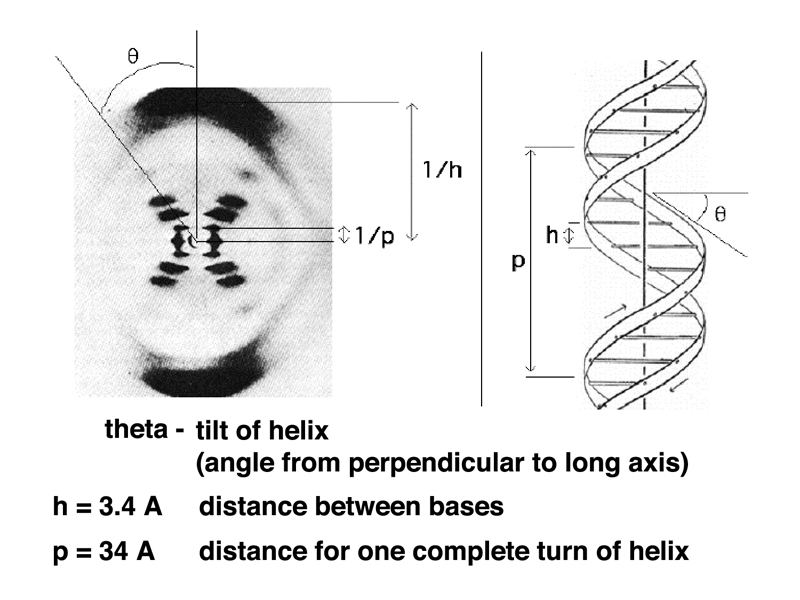
While Rosalind Franklin was carrying out this work with Maurice Wilkins at King's College London, a graduate student, James Watson, was working with physicist Francis Crick at the Cavendish Laboratory in Cambridge. Watson and Crick did not carry out experiments, but spent a great deal of time talking, reading, and attending scientific presentations, including several by Rosalind Franklin. Waton and Crick were attempting to build models of DNA.
One of their early models was a triple helix with the bases on the outside. They were visited by Maurice Wilkins and Rosalind Franklin. Franklin gave Watson and Crick a very direct refutation of their model before returning to London.
Franklin and Maurice Wilkins had a difficult relationship due to a misunderstanding of the terms of Franklin's employment. Upon arrival in London, Franklin had rebuilt the X-ray diffraction apparatus in Wilkin's lab, and began working with a graduate student, while Wilkins was away for several months. Wilkins returned to find the very productive Franklin under the impression that she was independent rather than an assistant to Wilkins. Thereafter, Wilkins retreated from most contact with Franklin, but had access to her data.
Wilkins shared Photo 51 with Watson and Crick, which immediately led them to formulate the double helix model of DNA. Besides the data from Photo 51, they were guided by the Chargaff Rules and the realization that an A:T base pair and a C:G base pair could be held together by hydrogen bonds and were approximately the same diameter.
When they showed their model to Rosalind Franklin, she immediately realized that it was correct, without knowing that key elements of the model were based on her data. Watson and Crick wrote a one-page paper for Nature in 1953, with Franklin writing an accompanying paper. Rosalind Franklin died of ovarian cancer in 1958, never knowing that she had been an involuntary and unacknowledged collaborator with Watson and Crick.
Watson, Crick, and Wilkins shared the Nobel Prize in 1962 for the determination of the secondary structure of DNA. The Nobel Prize is not awarded posthumously, so Rosalind Franklin's contribution was not acknowledged. Watson wrote a very self-serving memoir of the work, The Double Helix, which was published in 1968. Due to its many inaccuracies, in particular its treatment of Rosalind Franklin, Harvard University Press refused to publish the work after giving Wilkins, Crick, and others a chance to read the manuscript. It was published by a popular publisher and became a best seller.
The model for the secondary structure of DNA built by Watson and Crick has the features described below. The first is the idea of base pairing, shown below.
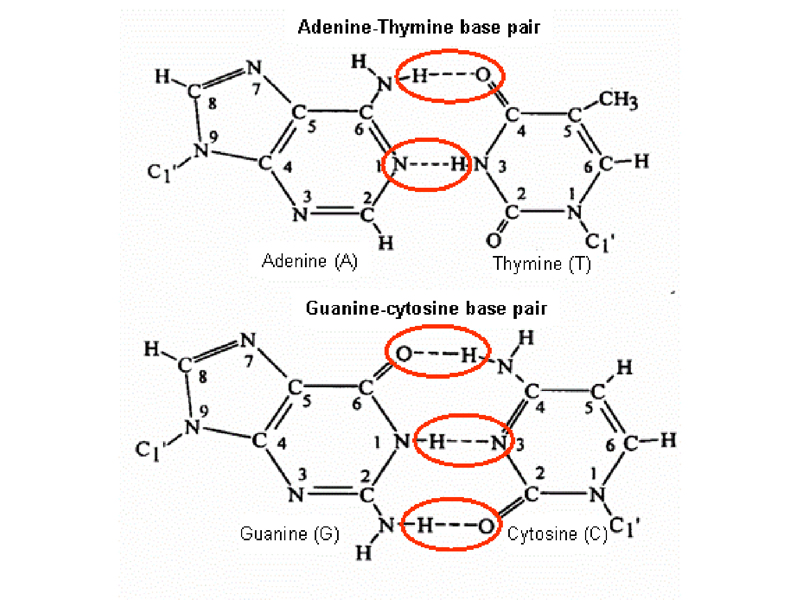
Notice that an A:T base pair is held together by two hydrogen bonds, while a G:C base pair is held together by three hydrogen bonds. This has consequences that we will explore later.
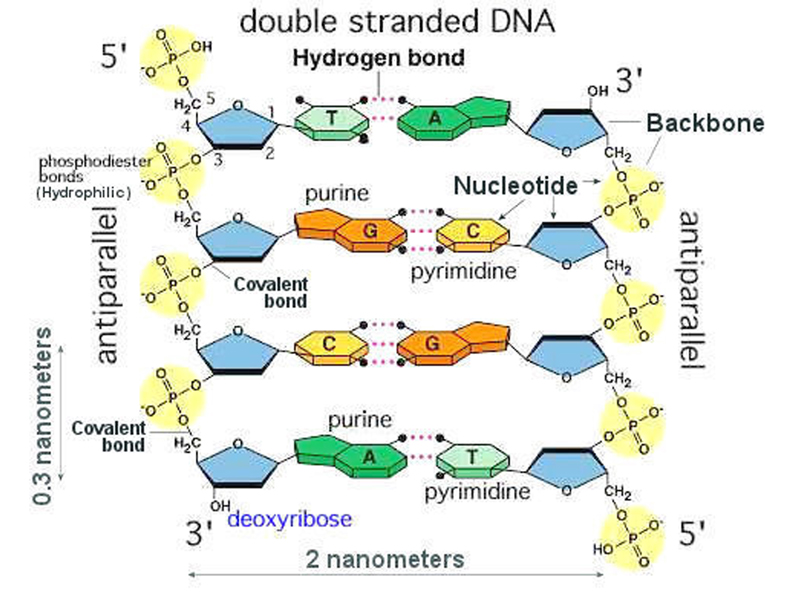
The figure below shows base pairing in the context of the double helix. Note that the two sugar-phosphate backbones are antiparallel: the one on the left runs from 5' to 3', while the one on the right runs 3' to 5'.
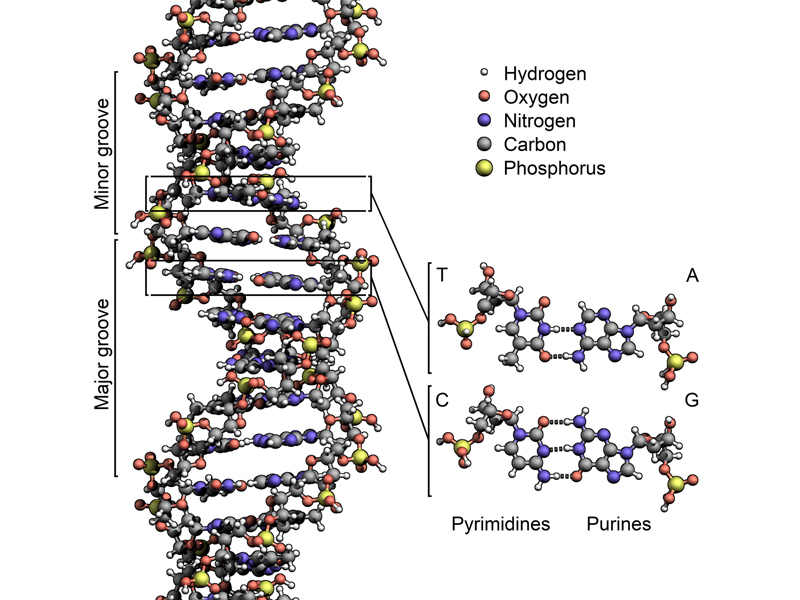
The figure below shows a more accurate model incorporating all of these features.
Because the paired bases are only held together by weak hydrogen bonds, the two strands of DNA are easily separated by raising the temperature. Because of base pairing, each strand has all of the information of the original molecule, suggesting a mechanism for replication. Watson and Crick wanted to make sure that everyone knew that they understood this, so they included in their Nature paper one of the great lines of the scientific literature:
"It has not escaped our notice that the specific pairing we have postulated immediately suggests a possible copying mechanism for the genetic material."
With it is obvious that base pairing provides a basis for replication of DNA, we can imagine several ways in which this might occur, shown in the drawing below.
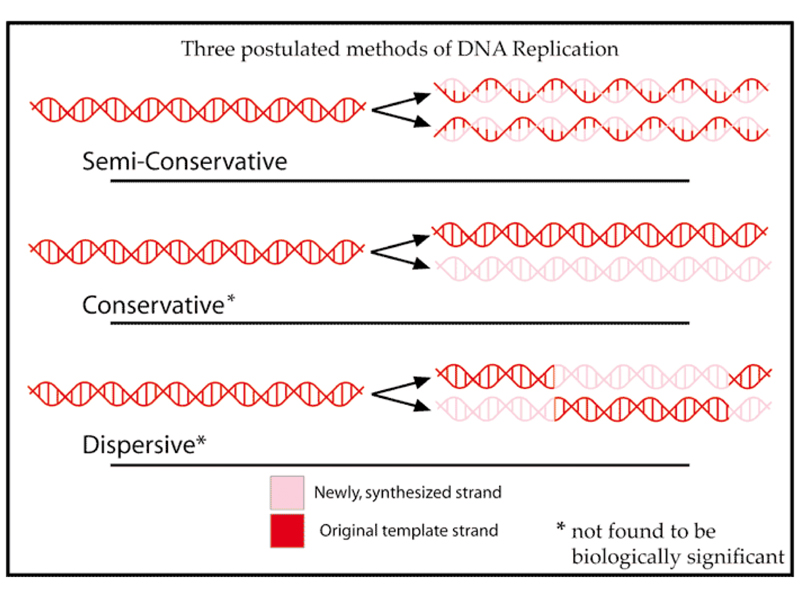
DNA replication might be semi-conservative, meaning that during replication, the strands separate, each serving as a template for the synthesis of a new strand. Newly replicated DNA would consist of one old and one new strand in this model.
DNA replication might be conservative, with the two old strands being copied into a new molecule with two new strands.
DNA replication might be dispersive, with conservative replication occurring in regions of the molecule to generate two new molecules that are chimeras of two old strands and two new strands.
In 1958, Mat Meselson and Frank Stahl, two graduate students at Cal Tech, carried out an experiment to determine which of these models for DNA replication was correct. They grew E. coli for many generations in a medium containing heavy nirtogen (15N), then innoculated the heavy cells into a large quantity of fresh medium containing normal nitrogen (14N). They took samples at various timepoints to obtain DNA that had not yet replicated, DNA that had replicated once, and DNA that had replicated more than once.
They subjected their samples to ultracentrifugation in a solution of cesium chloride (CsCl). Cesium is so dense that at high speeds, the cesium ions begin to settle to the bottom of the tube, generating a density gradient along the length of the tube. DNA will form a band at its buoyant density, allowing DNA that is fully heavy to be distinguished from DNA that is partially heavy or fully light. Their results are shown in the image below.
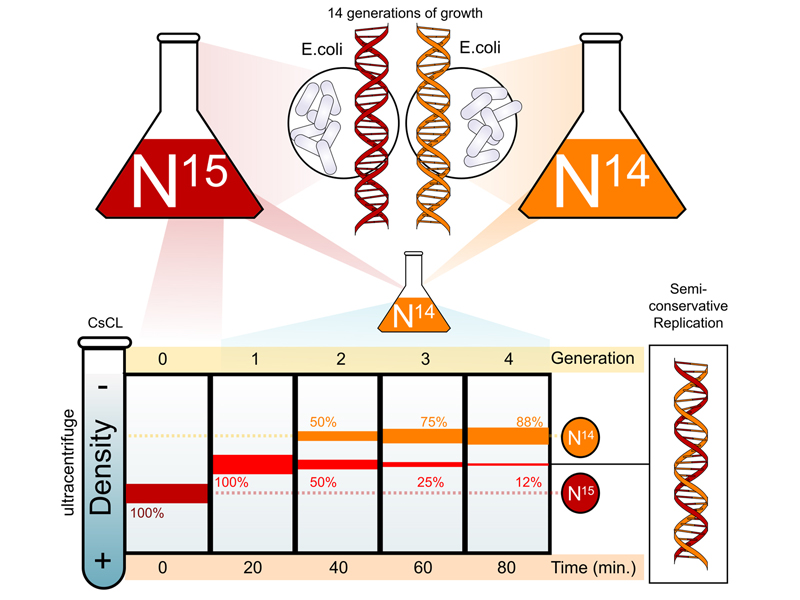
At time zero, we see that all of the DNA is fully heavy. After a single round of replication at 20 minutes, all of the DNA bands at a position indicating that it is half heavy, consisting of one heavy strand and one light strand. After a second round of replication at 40 minutes, we see that half of the DNA is fully light and half is half heavy. With each additional round of replication, we see an increase in the amount of fully light DNA and a decrease in the amount of half heavy DNA.
The results confirm that DNA replication is semiconservative. Satisfy yourself that this is the case by working through what the results would look like if DNA replication were conservative or dispersive.